Experiment 5.1:
Aim: To study the factors affecting the rate of evaporation of water.
(A) Humidity:
Problem statement: Does humidity affect the rate of evaporation of water?
Hypothesis: The higher the humidity, the lower the rate of evaporation of water.
Variables:
(a) Constant variables: Surrounding temperature, volume of water, movement of air and exposed surface area of water
(b) Manipulated variable: Humidity
(c) Responding variable: Rate of evaporation of water
Materials: Anhydrous cobalt chloride papers, water, thread and anhydrous calcium chloride
Apparatus: Bell jar and beaker
Procedure:
1. Dip two anhydrous cobalt chloride papers into water until completely wet.
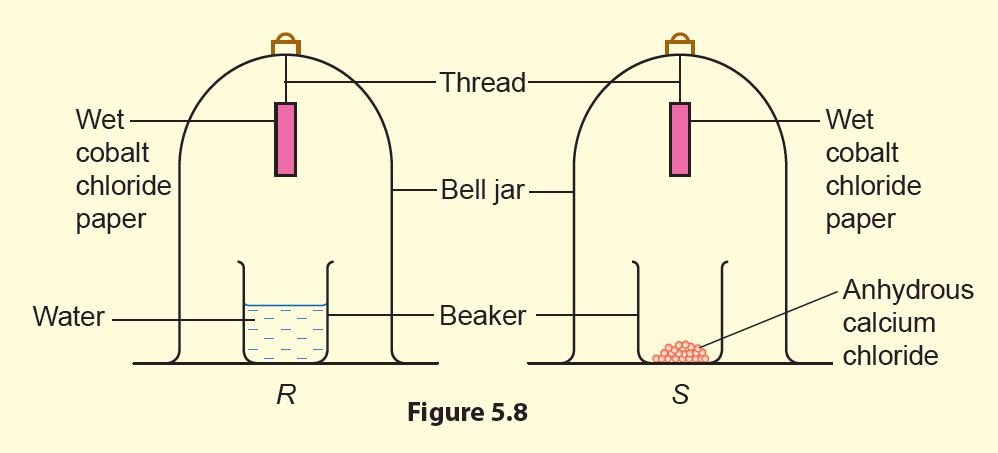
2. Set up the apparatus as shown in Figure 5.8.
3. Observe the cobalt chloride papers.
4. Record your observations in a table.
Aim: To study the factors affecting the rate of evaporation of water.
(A) Humidity:
Problem statement: Does humidity affect the rate of evaporation of water?
Hypothesis: The higher the humidity, the lower the rate of evaporation of water.
Variables:
(a) Constant variables: Surrounding temperature, volume of water, movement of air and exposed surface area of water
(b) Manipulated variable: Humidity
(c) Responding variable: Rate of evaporation of water
Materials: Anhydrous cobalt chloride papers, water, thread and anhydrous calcium chloride
Apparatus: Bell jar and beaker
Procedure:
1. Dip two anhydrous cobalt chloride papers into water until completely wet.
2. Set up the apparatus as shown in Figure 5.8.
3. Observe the cobalt chloride papers.
4. Record your observations in a table.

(B) Surrounding temperature:
Problem statement: Does surrounding temperature affect the rate of evaporation of water?
Hypothesis: The higher the temperature of surrounding, the higher the rate of evaporation of water
Variables:
(a) Constant variables: Humidity, volume of air, movement of air, exposed surface area of water
(b) Manipulated variable: Surrounding temperature
(c) Responding variable: Rate of evaporation of water
Materials: Anhydrous cobalt chloride papers and water
Apparatus: Filament lamp and white tile
Procedure:
1. Label two anhydrous cobalt chloride papers as J and K.
2. Dip both papers, J and K in water until completely wet.
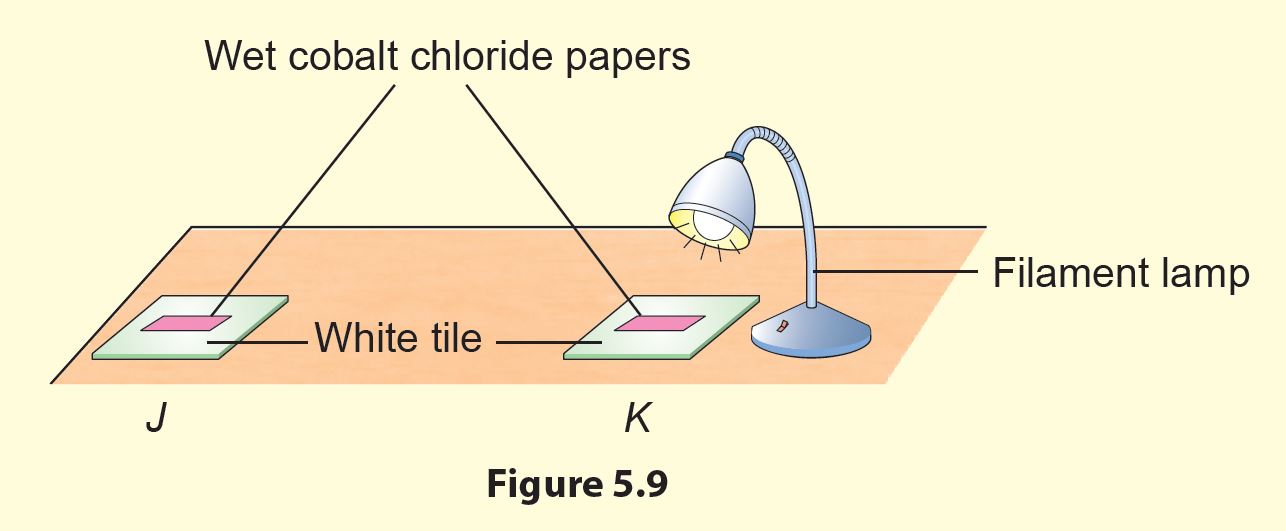
3. Place papers J and K on a table as shown in Figure 5.9.
4. Observe the cobalt chloride papers.
5. Record your observations in a table.
Problem statement: Does surrounding temperature affect the rate of evaporation of water?
Hypothesis: The higher the temperature of surrounding, the higher the rate of evaporation of water
Variables:
(a) Constant variables: Humidity, volume of air, movement of air, exposed surface area of water
(b) Manipulated variable: Surrounding temperature
(c) Responding variable: Rate of evaporation of water
Materials: Anhydrous cobalt chloride papers and water
Apparatus: Filament lamp and white tile
Procedure:
1. Label two anhydrous cobalt chloride papers as J and K.
2. Dip both papers, J and K in water until completely wet.
3. Place papers J and K on a table as shown in Figure 5.9.
4. Observe the cobalt chloride papers.
5. Record your observations in a table.

(C) Exposed surface area of water:
Problem statement: Does exposed surface area of water affect the rate of evaporation of water?
Hypothesis: The larger the exposed surface area of water, the higher the rate of water evaporation.
Variables:
(a) Constant variables: Humidity, volume of air, movement of air and surrounding temperature
(b) Manipulated variable: Exposed surface area of water
(c) Responding variable: Rate of evaporation of water
Materials: Filter papers, water and thread
Apparatus: Retort stand with clamp
Procedure:
1. Prepare three filter papers, P, Q and R.
2. Dip all the three filter papers in water.
3. Fold filter paper Q into two and filter paper R into four.
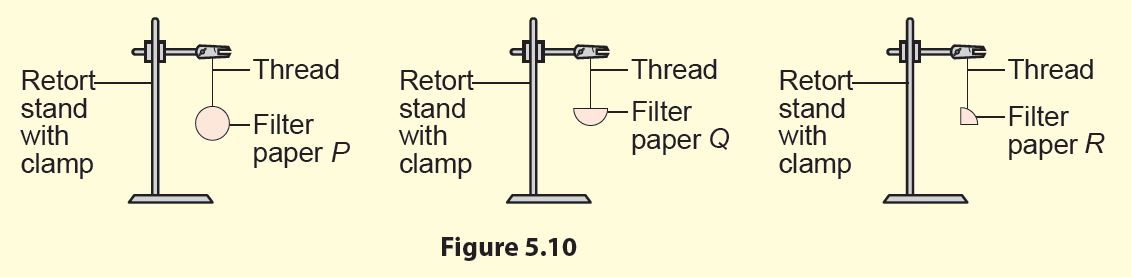
4. Hang all the three filter papers on different retort stands (Figure 5.10).
5. Record the time taken for the filter papers to dry in a table.
Problem statement: Does exposed surface area of water affect the rate of evaporation of water?
Hypothesis: The larger the exposed surface area of water, the higher the rate of water evaporation.
Variables:
(a) Constant variables: Humidity, volume of air, movement of air and surrounding temperature
(b) Manipulated variable: Exposed surface area of water
(c) Responding variable: Rate of evaporation of water
Materials: Filter papers, water and thread
Apparatus: Retort stand with clamp
Procedure:
1. Prepare three filter papers, P, Q and R.
2. Dip all the three filter papers in water.
3. Fold filter paper Q into two and filter paper R into four.
4. Hang all the three filter papers on different retort stands (Figure 5.10).

5. Record the time taken for the filter papers to dry in a table.
(D) Movement of air:
Problem statement: Does movement of air affect the rate of evaporation of water?
Hypothesis: The faster the movement of air, the higher the rate of evaporation of water.
Variables:
(a) Constant variables: Humidity, volume of water, exposed surface area of water and surrounding temperature
(b) Manipulated variable: Movement of air
(c) Responding variable: Rate of evaporation of water
Materials: Anhydrous cobalt chloride papers, cellophane tape and water
Apparatus: Microscope slides, fan and dropper
Procedure:
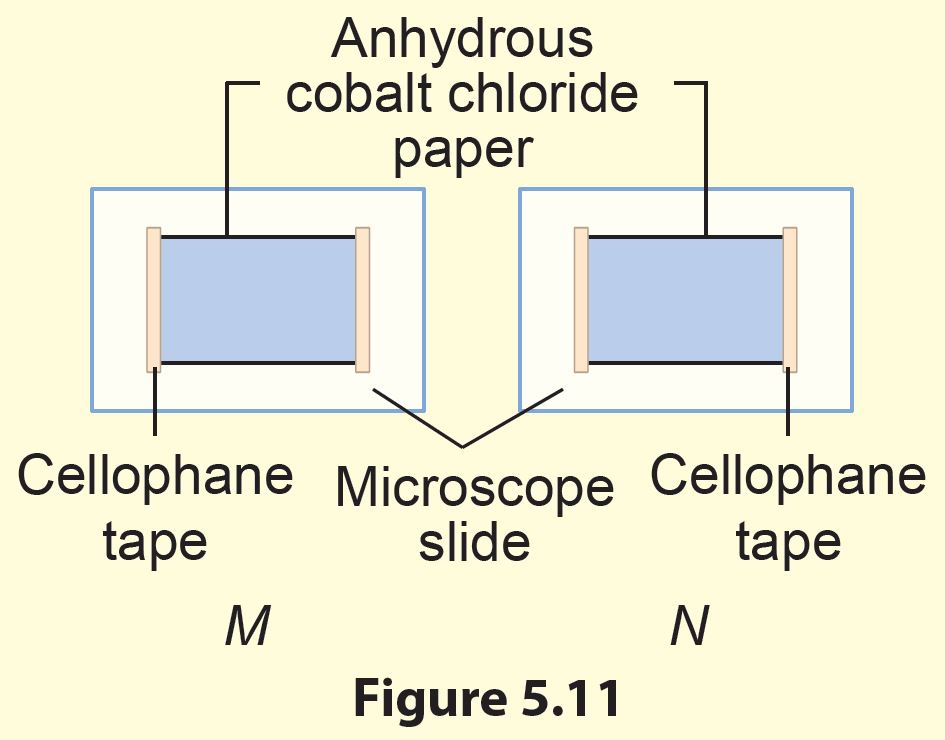
1. Stick two anhydrous cobalt chloride papers on microscope slides using cellophane tape and label them as M and N (Figure 5.11).
2. Add a few drops of water on each cobalt chloride paper.
3. Place slide M under a moving fan and slide N away from the fan.
4. Record your observations after 15 minutes in a table.
Conclusion:
Is the hypothesis for each experiment accepted? Give your reasons.
Problem statement: Does movement of air affect the rate of evaporation of water?
Hypothesis: The faster the movement of air, the higher the rate of evaporation of water.
Variables:
(a) Constant variables: Humidity, volume of water, exposed surface area of water and surrounding temperature
(b) Manipulated variable: Movement of air
(c) Responding variable: Rate of evaporation of water
Materials: Anhydrous cobalt chloride papers, cellophane tape and water
Apparatus: Microscope slides, fan and dropper
Procedure:
1. Stick two anhydrous cobalt chloride papers on microscope slides using cellophane tape and label them as M and N (Figure 5.11).
2. Add a few drops of water on each cobalt chloride paper.
3. Place slide M under a moving fan and slide N away from the fan.
4. Record your observations after 15 minutes in a table.

Conclusion:
Is the hypothesis for each experiment accepted? Give your reasons.
Question:
1. State the functions of water and anhydrous calcium chloride in Experiment A.
2. What is the use of the filament lamp in Experiment B?
3. How does surface area affect the rate of evaporation of water?
4. Why is a fan used in Experiment D?
1. State the functions of water and anhydrous calcium chloride in Experiment A.
2. What is the use of the filament lamp in Experiment B?
3. How does surface area affect the rate of evaporation of water?
4. Why is a fan used in Experiment D?
Answer:
1. Water moisturises the air in bell jar R while anhydrous calcium chloride absorbs water vapour in bell jar S so that the air will become dry.
2. The filament lamp is used as a source of heat energy.
3. The larger the surface area exposed, the higher the rate of evaporation of water.
4. To increase the movement of air.
1. Water moisturises the air in bell jar R while anhydrous calcium chloride absorbs water vapour in bell jar S so that the air will become dry.
2. The filament lamp is used as a source of heat energy.
3. The larger the surface area exposed, the higher the rate of evaporation of water.
4. To increase the movement of air.