Activity 5.3:
Aim: To observe the effects of impurities on the melting point and boiling point of water.
Materials: Distilled water, ice cubes, two thick towels and table salt
Apparatus: Beaker, conical flasks, thermometer, spatula, Bunsen burner, tripod stand, wire gauze, two-hole rubber stopper, glass tube and stopwatch
(A) The effect of table salt on the melting point of ice
Instruction
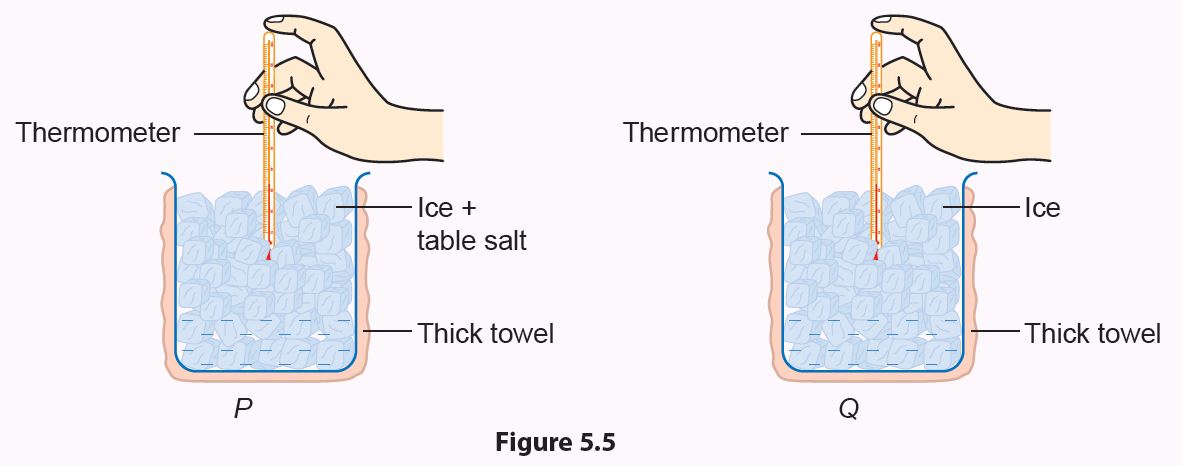
1. Wrap two similar-sized beakers in thick towels and label them as P and Q.
2. Add the same number of ice cubes into both beakers.
3. Add one spatula of salt into beaker P (Figure 5.5).
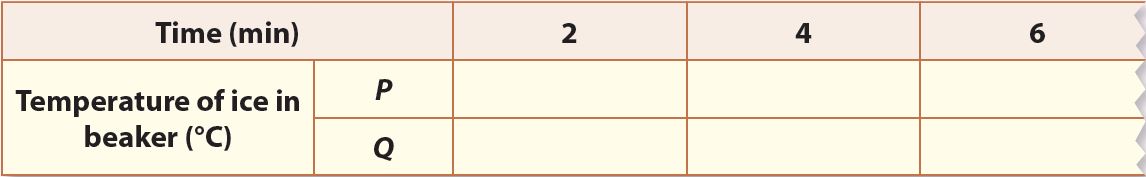
4. Record the temperature of the ice in both beakers every 2 minutes until the temperature becomes constant.
Observation
(B) The effect of table salt on the boiling point of water
Instruction
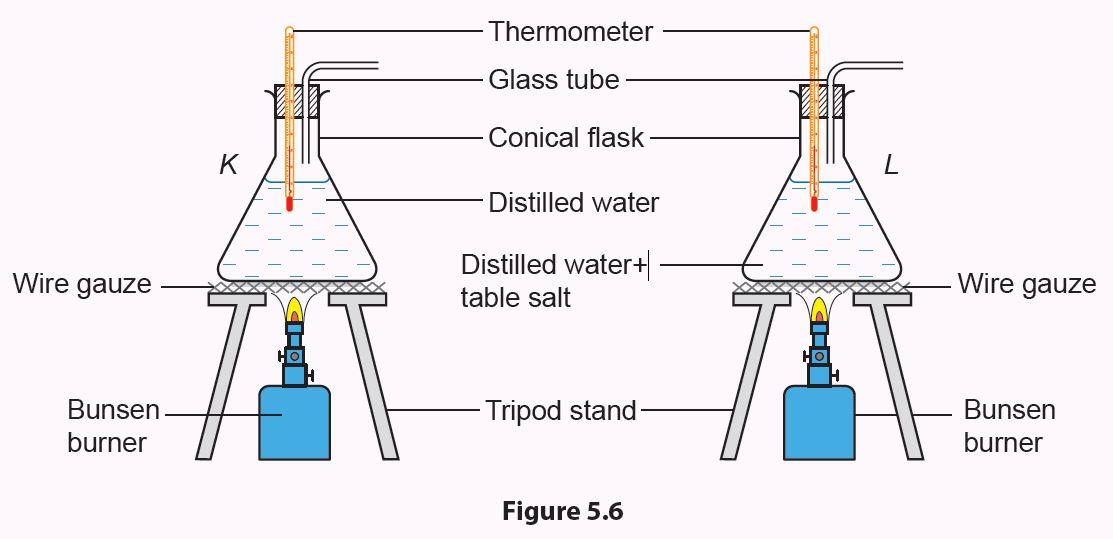
1. Set up the apparatus as shown in Figure 5.6 and label the conical flasks as K and L.
2. Heat the distilled water in both conical flasks until they reach 80°C.
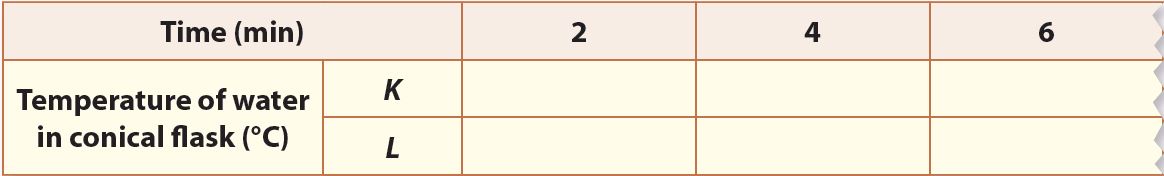
3. Start recording the temperature of the water in both conical flasks every 2 minutes until the temperature becomes constant.
Observation
Questions
1. Using the Kinetic Theory of Matter, explain the change in the state of ice in Activity A.
2. Give an inference for the boiling point of distilled water and the boiling point of distilled water mixed with salt.
3. What can be concluded about impurities from Activity A and B?
Answer:
1. Ice particles absorb heat energy from the surrounding and start to vibrate faster. The forces of attraction between the particles are overcome, causing the ice particles to move faster and randomly. Hence the state of matter of ice changes from solid to liquid.
2. The boiling point of distilled water is lower than the boiling point of distilled water mixed with salt because the presence of impurities (salt) increase the boiling point of water.
3. Impurities increase the boiling point and decrease the melting point.
Aim: To observe the effects of impurities on the melting point and boiling point of water.
Materials: Distilled water, ice cubes, two thick towels and table salt
Apparatus: Beaker, conical flasks, thermometer, spatula, Bunsen burner, tripod stand, wire gauze, two-hole rubber stopper, glass tube and stopwatch
(A) The effect of table salt on the melting point of ice
Instruction
1. Wrap two similar-sized beakers in thick towels and label them as P and Q.
2. Add the same number of ice cubes into both beakers.
3. Add one spatula of salt into beaker P (Figure 5.5).
4. Record the temperature of the ice in both beakers every 2 minutes until the temperature becomes constant.

Observation

(B) The effect of table salt on the boiling point of water
Instruction
1. Set up the apparatus as shown in Figure 5.6 and label the conical flasks as K and L.
2. Heat the distilled water in both conical flasks until they reach 80°C.
3. Start recording the temperature of the water in both conical flasks every 2 minutes until the temperature becomes constant.

Observation

Questions
1. Using the Kinetic Theory of Matter, explain the change in the state of ice in Activity A.
2. Give an inference for the boiling point of distilled water and the boiling point of distilled water mixed with salt.
3. What can be concluded about impurities from Activity A and B?
Answer:
1. Ice particles absorb heat energy from the surrounding and start to vibrate faster. The forces of attraction between the particles are overcome, causing the ice particles to move faster and randomly. Hence the state of matter of ice changes from solid to liquid.
2. The boiling point of distilled water is lower than the boiling point of distilled water mixed with salt because the presence of impurities (salt) increase the boiling point of water.
3. Impurities increase the boiling point and decrease the melting point.