Activity 6.1:
Aim: To study the properties of acids and alkalis.
Materials: Dilute hydrochloric acid, concentrated hydrochloric acid, dilute sodium hydroxide solution, concentrated sodium hydroxide solution, lime juice, bitter gourd juice, magnesium ribbon, filter paper, sandpaper, blue litmus paper and red litmus paper, wooden splinter, matches, pH paper and pH chart
Apparatus: Test tube, dropper, Petri dish and white tile
Instruction
(A) pH value
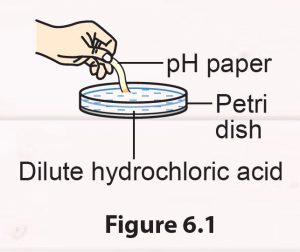
1. Put 10 drops of dilute hydrochloric acid in a Petri dish.
2. Test the substance in the Petri dish with a piece of pH paper (Figure 6.1).
3. Determine the pH value by comparing the colour of the pH paper with a pH chart.
4. Record your observation.
5. Repeat steps 1 to 4 by replacing dilute hydrochloric acid with dilute sodium hydroxide solution.
(B) Taste
1. Taste lime juice followed by bitter gourd juice. Gargle with water after tasting each substance.
2. Record your observations.
(C) Corrosiveness (Teacher’s demonstration)
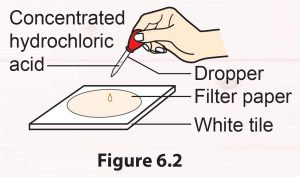
1. Put one drop of concentrated hydrochloric acid on a piece of filter paper placed on a white tile (Figure 6.2).
2. Record your observation.
3. Repeat steps 1 and 2 by replacing concentrated hydrochloric acid with concentrated sodium hydroxide solution.
(D) Effect on blue litmus paper and red litmus paper
1. Place a blue litmus paper and a red litmus paper on a white tile.
2. Put one drop of dilute hydrochloric acid on both litmus papers and record your observations.
3. Repeat steps 1 and 2 by replacing dilute hydrochloric acid with dilute sodium hydroxide solution.
(E) Reaction with metals
1. Clean a magnesium ribbon with sandpaper.
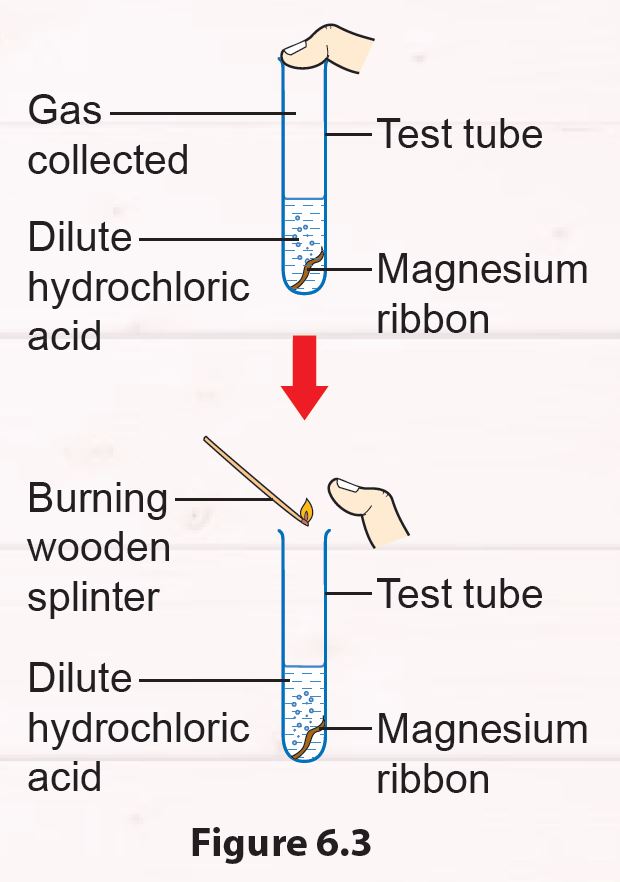
2. Put the magnesium ribbon into a test tube filled with 5 ml of dilute hydrochloric acid.
3. Close the test tube with your thumb for one minute.
4. Remove your thumb and place a lighted wooden splinter at the mouth of the test tube (Figure 6.3).
5. Record your observation.
6. Repeat steps 1 to 5 by replacing dilute hydrochloric acid with dilute sodium hydroxide solution.
Observation
Questions
1. What is the pH range of alkalis?
2. Give an inference for your observation in Activity E.
3. Why should the magnesium ribbon be cleaned with sandpaper before using it?
4. Predict the taste of vinegar and neem.
5. Give the operational definition of an acid and an alkali.
Answer:
1. pH 8 – pH 14
2. The reaction between acid and metal releases hydrogen gas because acid contains hydrogen ions.
3. Magnesium ribbon exposed to air will be oxidised. Therefore, the magnesium ribbon should be cleaned with sandpaper to remove the oxidised parts.
4.
Vinegar – Sour
Neem – Bitter
5. An acid is a chemical that turns a blue litmus paper red.
An alkali is a chemical that turns a red litmus paper blue.
(Any other answers are accepted)
Aim: To study the properties of acids and alkalis.
Materials: Dilute hydrochloric acid, concentrated hydrochloric acid, dilute sodium hydroxide solution, concentrated sodium hydroxide solution, lime juice, bitter gourd juice, magnesium ribbon, filter paper, sandpaper, blue litmus paper and red litmus paper, wooden splinter, matches, pH paper and pH chart
Apparatus: Test tube, dropper, Petri dish and white tile
Instruction
(A) pH value
1. Put 10 drops of dilute hydrochloric acid in a Petri dish.
2. Test the substance in the Petri dish with a piece of pH paper (Figure 6.1).
3. Determine the pH value by comparing the colour of the pH paper with a pH chart.
4. Record your observation.
5. Repeat steps 1 to 4 by replacing dilute hydrochloric acid with dilute sodium hydroxide solution.

(B) Taste
1. Taste lime juice followed by bitter gourd juice. Gargle with water after tasting each substance.
2. Record your observations.
(C) Corrosiveness (Teacher’s demonstration)
1. Put one drop of concentrated hydrochloric acid on a piece of filter paper placed on a white tile (Figure 6.2).
2. Record your observation.
3. Repeat steps 1 and 2 by replacing concentrated hydrochloric acid with concentrated sodium hydroxide solution.
(D) Effect on blue litmus paper and red litmus paper
1. Place a blue litmus paper and a red litmus paper on a white tile.
2. Put one drop of dilute hydrochloric acid on both litmus papers and record your observations.
3. Repeat steps 1 and 2 by replacing dilute hydrochloric acid with dilute sodium hydroxide solution.

(E) Reaction with metals
1. Clean a magnesium ribbon with sandpaper.
2. Put the magnesium ribbon into a test tube filled with 5 ml of dilute hydrochloric acid.
3. Close the test tube with your thumb for one minute.
4. Remove your thumb and place a lighted wooden splinter at the mouth of the test tube (Figure 6.3).
5. Record your observation.
6. Repeat steps 1 to 5 by replacing dilute hydrochloric acid with dilute sodium hydroxide solution.

Observation

Questions
1. What is the pH range of alkalis?
2. Give an inference for your observation in Activity E.
3. Why should the magnesium ribbon be cleaned with sandpaper before using it?
4. Predict the taste of vinegar and neem.
5. Give the operational definition of an acid and an alkali.
Answer:
1. pH 8 – pH 14
2. The reaction between acid and metal releases hydrogen gas because acid contains hydrogen ions.
3. Magnesium ribbon exposed to air will be oxidised. Therefore, the magnesium ribbon should be cleaned with sandpaper to remove the oxidised parts.
4.
Vinegar – Sour
Neem – Bitter
5. An acid is a chemical that turns a blue litmus paper red.
An alkali is a chemical that turns a red litmus paper blue.
(Any other answers are accepted)