Activity 6.5:
Aim: To study the neutralisation reaction between hydrochloric acid and sodium hydroxide solution.
Materials: Phenolphthalein, 0.5 M hydrochloric acid and 0.5 M sodium hydroxide solution
Apparatus: Burette, pipette, conical flask, retort stand with clamp, white tile and filter funnel
Instruction
1. Fill 30 ml of hydrochloric acid into a burette using a filter funnel and record the initial reading of the burette.
2. Transfer 25 ml of sodium hydroxide solution into a conical flask using a pipette.
3. Add three drops of phenolphthalein into the conical flask.
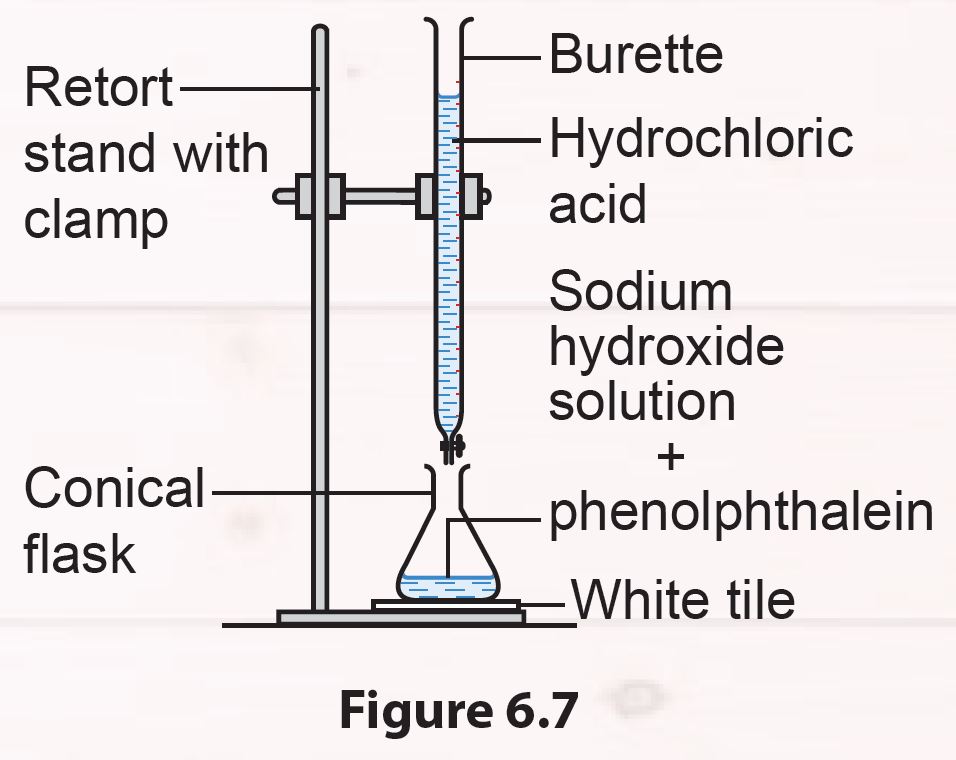
Set up the apparatus as shown in Figure 6.7.
4. Add the hydrochloric acid from the burette drop by drop into the conical flask while shaking the flask gently.
5. Stop adding the acid when the sodium hydroxide solution changes colour from pink to colourless.
6. Record the final reading of the burette.
Observation

Questions
1. What is the volume of hydrochloric acid required to neutralise 25 ml of sodium hydroxide solution?
2. Write the word equation for the reaction between the acid and alkali in this activity.
Answer:
1. (Answer based on the student’s finding)
2. Hydrochloric acid + Sodium hydroxide ➝ Sodium chloride + Water
Aim: To study the neutralisation reaction between hydrochloric acid and sodium hydroxide solution.
Materials: Phenolphthalein, 0.5 M hydrochloric acid and 0.5 M sodium hydroxide solution
Apparatus: Burette, pipette, conical flask, retort stand with clamp, white tile and filter funnel
Instruction
1. Fill 30 ml of hydrochloric acid into a burette using a filter funnel and record the initial reading of the burette.
2. Transfer 25 ml of sodium hydroxide solution into a conical flask using a pipette.
3. Add three drops of phenolphthalein into the conical flask.
Set up the apparatus as shown in Figure 6.7.
4. Add the hydrochloric acid from the burette drop by drop into the conical flask while shaking the flask gently.
5. Stop adding the acid when the sodium hydroxide solution changes colour from pink to colourless.
6. Record the final reading of the burette.

Observation

Questions
1. What is the volume of hydrochloric acid required to neutralise 25 ml of sodium hydroxide solution?
2. Write the word equation for the reaction between the acid and alkali in this activity.
Answer:
1. (Answer based on the student’s finding)
2. Hydrochloric acid + Sodium hydroxide ➝ Sodium chloride + Water