Activity 5.2:
Aim: To determine the composition of elements in a water molecule.
Materials: Distilled water, dilute hydrochloric acid, wooden splinters and matches
Apparatus: Electrolysis cell, switch, two measuring cylinders, dropper and crocodile clips
Instruction
1. Label two measuring cylinders as K and L.
2. Set up the apparatus as shown in Figure 5.4 by adding a few drops of dilute hydrochloric acid to the distilled water.
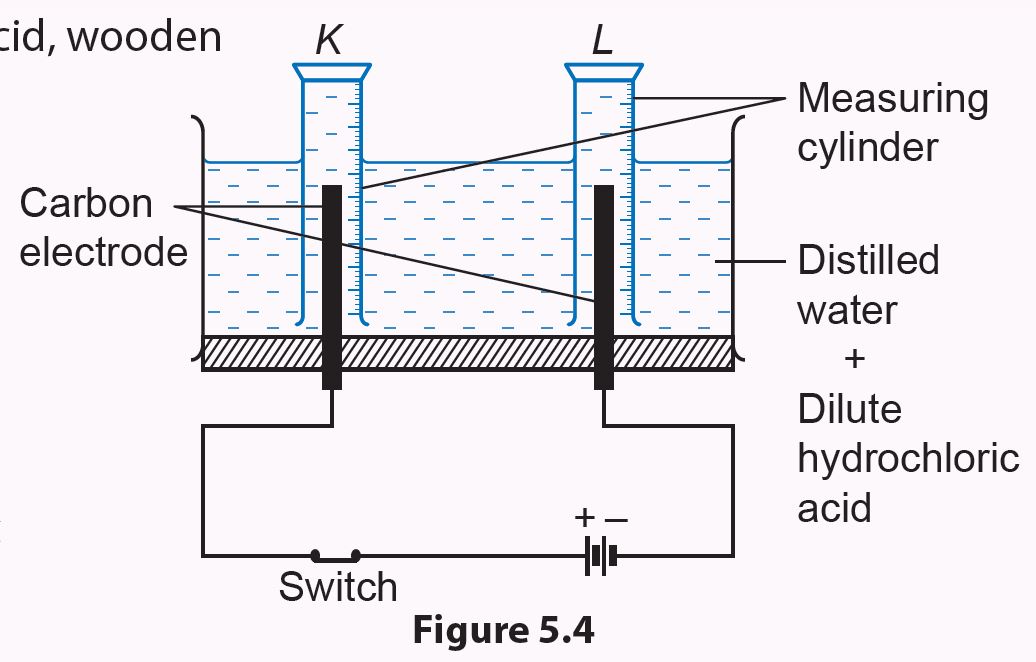
3. Connect the switch for 10 minutes.
4. Observe the changes that occur at both measuring cylinders.
5. After 10 minutes, turn off the switch and record the volumes of gas in each measuring cylinder.
6. Test the gases collected using wooden splinters.
(a) The gas in measuring cylinder K is tested with a glowing wooden splinter
(b) The gas in measuring cylinder L is tested with a burning wooden splinter
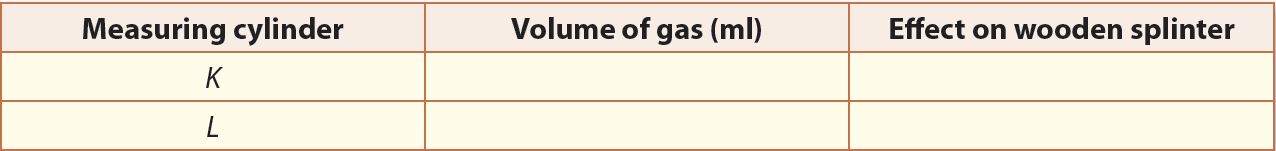
7. Record all your observations in a table.
Observation
Questions
1. Name the gases collected in measuring cylinders K and L.
2. (a) What is the ratio of the volume of gas in measuring cylinder K to L?
(b) Give an inference for your answer in 2 (a).
3. Why is dilute hydrochloric acid added into the distilled water?
Answer:
1. K – Oxygen
L – Hydrogen
2. (a) The ratio of gas K to gas L is 1:2
(b) One molecule of water is made up of one oxygen atom and two hydrogen atoms.
3. Dilute hydrochloric acid allows the current to flow better through water because water is a poor conductor of electricity.
Aim: To determine the composition of elements in a water molecule.
Materials: Distilled water, dilute hydrochloric acid, wooden splinters and matches
Apparatus: Electrolysis cell, switch, two measuring cylinders, dropper and crocodile clips
Instruction
1. Label two measuring cylinders as K and L.
2. Set up the apparatus as shown in Figure 5.4 by adding a few drops of dilute hydrochloric acid to the distilled water.

3. Connect the switch for 10 minutes.
4. Observe the changes that occur at both measuring cylinders.
5. After 10 minutes, turn off the switch and record the volumes of gas in each measuring cylinder.
6. Test the gases collected using wooden splinters.
(a) The gas in measuring cylinder K is tested with a glowing wooden splinter
(b) The gas in measuring cylinder L is tested with a burning wooden splinter
7. Record all your observations in a table.
Observation

Questions
1. Name the gases collected in measuring cylinders K and L.
2. (a) What is the ratio of the volume of gas in measuring cylinder K to L?
(b) Give an inference for your answer in 2 (a).
3. Why is dilute hydrochloric acid added into the distilled water?
Answer:
1. K – Oxygen
L – Hydrogen
2. (a) The ratio of gas K to gas L is 1:2
(b) One molecule of water is made up of one oxygen atom and two hydrogen atoms.
3. Dilute hydrochloric acid allows the current to flow better through water because water is a poor conductor of electricity.