Activity 7.3:
Aim: To prove that fuel, oxygen and heat are needed for combustion
(I) Fuel is needed for combustion
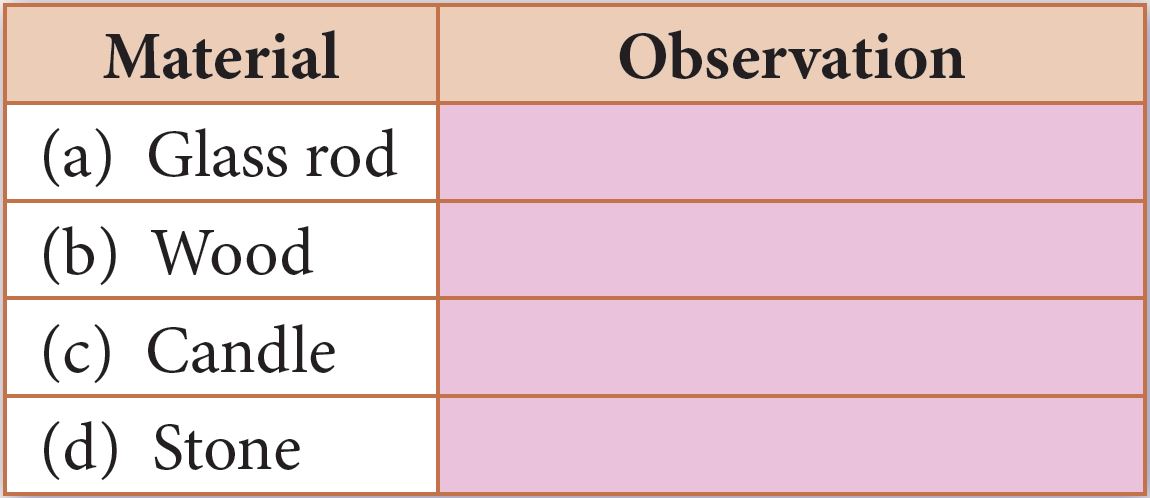
Materials and apparatus: Bunsen burner, tongs, lighter, glass rod, wood, candle, stone
Instruction
1. Light a Bunsen burner.
2. Hold a glass rod over the fire by using tongs.
3. Observe whether or not the glass rod burns.
4. Record your observation in a table.
5. Repeat steps 2 to 4 by using wood, candle and stone.
Questions
1. Based on your observation, classify the materials into fuel and non-fuel.
2. What is the conclusion that you can make from this activity?
Answer:
(A)
1. Fuel: wood and candle
Non-fuel: glass rod and stone
2.
Wood and candle are fuel while glass rod and stone are non-fuel
Aim: To prove that fuel, oxygen and heat are needed for combustion
(I) Fuel is needed for combustion
Materials and apparatus: Bunsen burner, tongs, lighter, glass rod, wood, candle, stone
Instruction
1. Light a Bunsen burner.
2. Hold a glass rod over the fire by using tongs.
3. Observe whether or not the glass rod burns.
4. Record your observation in a table.
5. Repeat steps 2 to 4 by using wood, candle and stone.

Questions
1. Based on your observation, classify the materials into fuel and non-fuel.
2. What is the conclusion that you can make from this activity?
Answer:
(A)
1. Fuel: wood and candle
Non-fuel: glass rod and stone
2.
Wood and candle are fuel while glass rod and stone are non-fuel
(II) Oxygen is needed for combustion:
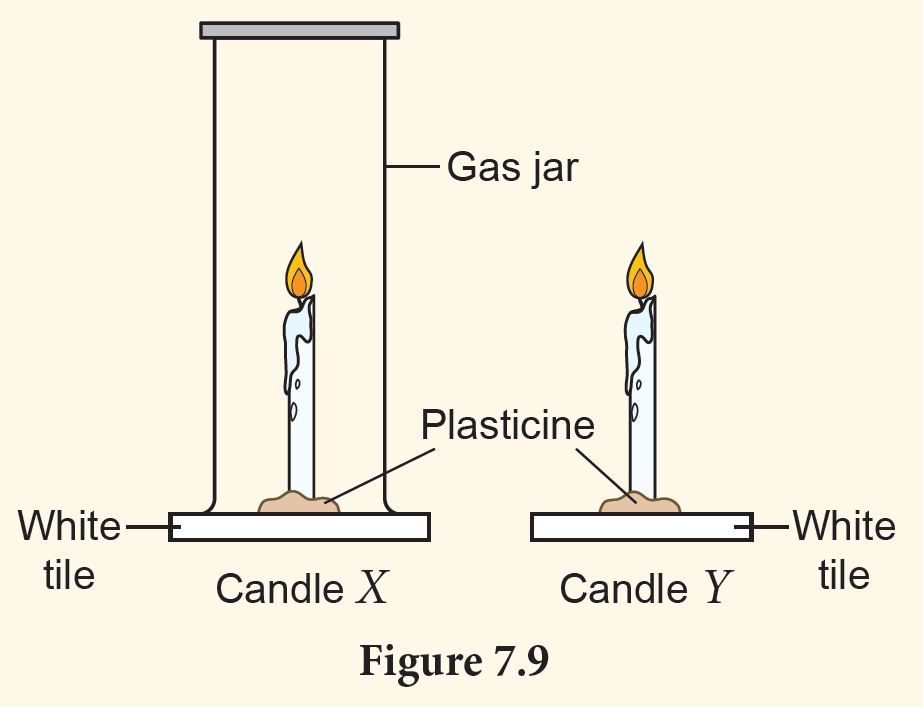
Materials and apparatus: Gas jar, two white tiles, two candles of the same size, plasticine, lighter
Instruction
1. Hold two candles of the same size on white tiles by using plasticine (Figure 7.9).
2. Light candles X and Y.
3. Turn a gas jar over candle X.
4. Observe which candle extinguishes first.
Questions
1. Which candle burns longer? Why?
2. What is the gas used during the burning of candle?
3. What is the conclusion that you can make from this activity?
Answer:
(B)
1. Candle Y because the candle obtains more oxygen compared to Candle X.
2. Oxygen
3. Oxygen is needed for combustion
Materials and apparatus: Gas jar, two white tiles, two candles of the same size, plasticine, lighter
Instruction
1. Hold two candles of the same size on white tiles by using plasticine (Figure 7.9).
2. Light candles X and Y.
3. Turn a gas jar over candle X.
4. Observe which candle extinguishes first.

Questions
1. Which candle burns longer? Why?
2. What is the gas used during the burning of candle?
3. What is the conclusion that you can make from this activity?
Answer:
(B)
1. Candle Y because the candle obtains more oxygen compared to Candle X.
2. Oxygen
3. Oxygen is needed for combustion
(III) Heat is needed for combustion:
Materials and apparatus: Match stick, match stick which has been stored inside the refrigerator, matchbox
Instruction
1. Label the match stick which has been stored inside the refrigerator as P and the other match stick as Q.
2. Light match sticks P and Q. Observe the changes that occur.
Question:
1. Do both match sticks ignite? Why?
2. What is the conclusion that you can make from this activity?
Answer:
(C)
1. Match P does not ignite due to lack of heat and match Q ignites because it has heat.
2. Heat is needed for combustion.
Materials and apparatus: Match stick, match stick which has been stored inside the refrigerator, matchbox
Instruction
1. Label the match stick which has been stored inside the refrigerator as P and the other match stick as Q.
2. Light match sticks P and Q. Observe the changes that occur.
Question:
1. Do both match sticks ignite? Why?
2. What is the conclusion that you can make from this activity?
Answer:
(C)
1. Match P does not ignite due to lack of heat and match Q ignites because it has heat.
2. Heat is needed for combustion.