Activity 7.1:
Aim: To determine the percentage of oxygen in the air
Materials and apparatus: Candle, plasticine, matches, glass basin, permanent marker pen, gas jar, gas jar stand, water.
Instruction
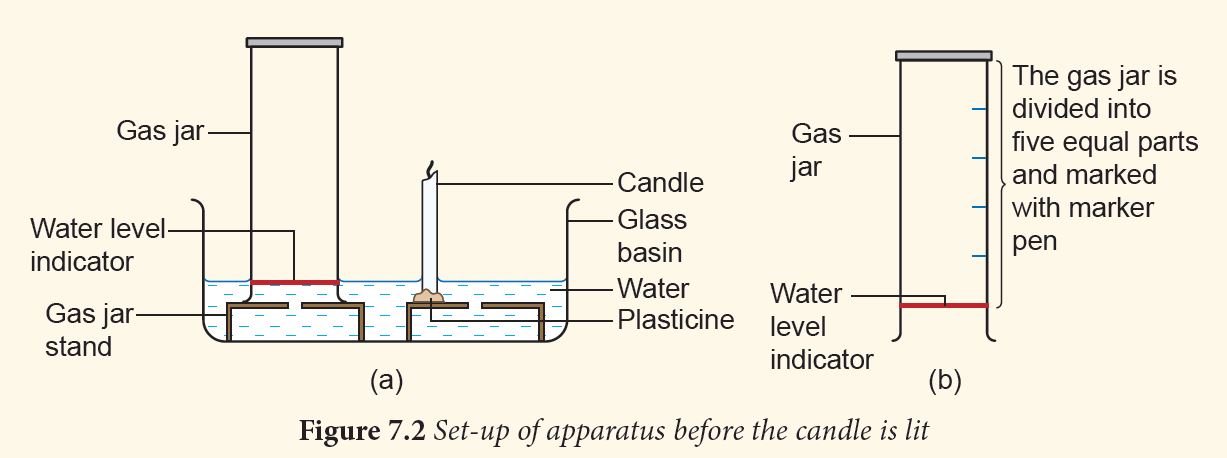
1. Prepare the apparatus as shown in Figure 7.2 (a).
2. Divide the gas jar into five equal parts (Figure 7.2 (b)).
3. Light the candle and invert the gas jar over the candle (Figure 7.3).
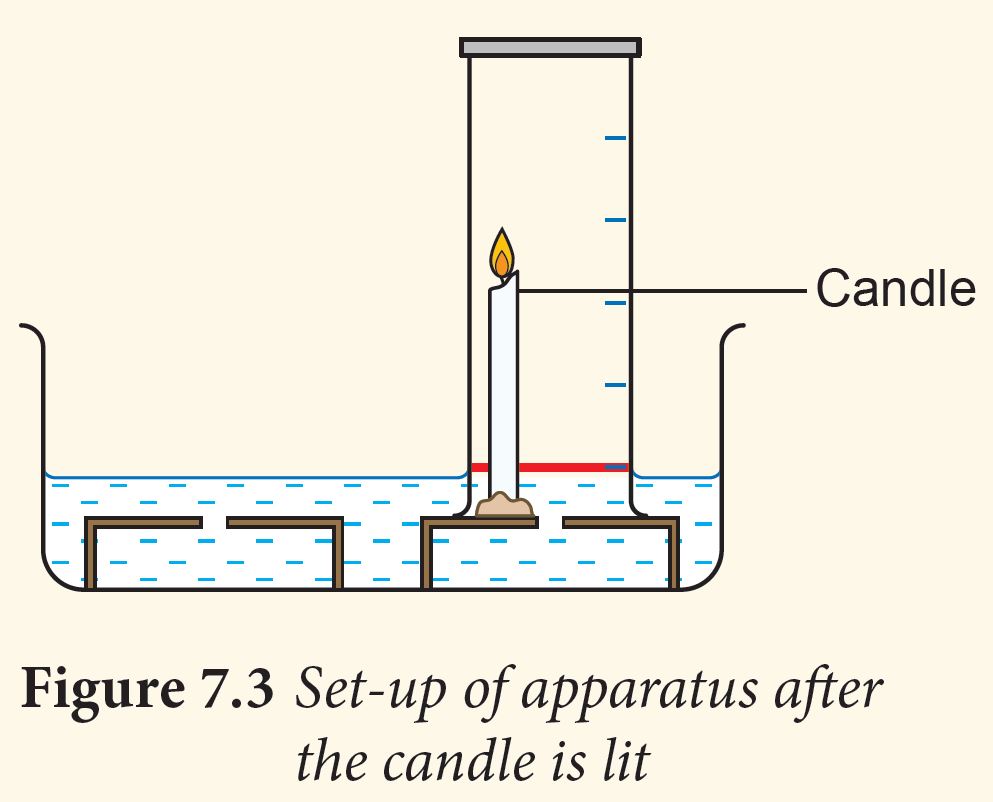
4. Observe and record the changes in the water level when the flame extinguishes.
5. Make a conclusion on the percentage of oxygen in the air.
Questions
1. What happened to the flame of the candle after a period of time? Why?
2. What happened to the water level at the end of this activity? Give reason(s) to the changes.
3. What is the percentage of oxygen in the air based on this activity?
Answer:
1. The flame is extinguished because all of the oxygen inside the jar has been used.
2. The water level increased because the water replaced the oxygen used for combustion.
3. 20%
Aim: To determine the percentage of oxygen in the air
Materials and apparatus: Candle, plasticine, matches, glass basin, permanent marker pen, gas jar, gas jar stand, water.
Instruction

1. Prepare the apparatus as shown in Figure 7.2 (a).
2. Divide the gas jar into five equal parts (Figure 7.2 (b)).
3. Light the candle and invert the gas jar over the candle (Figure 7.3).

4. Observe and record the changes in the water level when the flame extinguishes.
5. Make a conclusion on the percentage of oxygen in the air.
Questions
1. What happened to the flame of the candle after a period of time? Why?
2. What happened to the water level at the end of this activity? Give reason(s) to the changes.
3. What is the percentage of oxygen in the air based on this activity?
Answer:
1. The flame is extinguished because all of the oxygen inside the jar has been used.
2. The water level increased because the water replaced the oxygen used for combustion.
3. 20%