Activity 3.1:
Aim: To test for the presence of starch, glucose, protein and fat.
Materials: Iodine solution, 1% starch suspension, Benedict’s solution, 10% glucose solution, Millon’s reagent, albumen suspension, ethanol, cooking oil and distilled water
Apparatus: Test tube, dropper, beaker, Bunsen burner, wire gauze, tripod stand, test tube holder, stopper and test tube rack
(A) Iodine test for starch:
Instruction
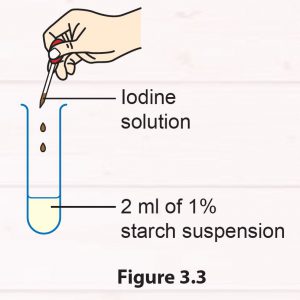
1. Pour 2 ml of starch suspension into a test tube.
2. Add two drops of iodine solution into the test tube (Figure 3.3).
3. Record your observations.
Aim: To test for the presence of starch, glucose, protein and fat.
Materials: Iodine solution, 1% starch suspension, Benedict’s solution, 10% glucose solution, Millon’s reagent, albumen suspension, ethanol, cooking oil and distilled water
Apparatus: Test tube, dropper, beaker, Bunsen burner, wire gauze, tripod stand, test tube holder, stopper and test tube rack
(A) Iodine test for starch:
Instruction
1. Pour 2 ml of starch suspension into a test tube.
2. Add two drops of iodine solution into the test tube (Figure 3.3).
3. Record your observations.

(B) Benedict’s test for glucose:
Instruction
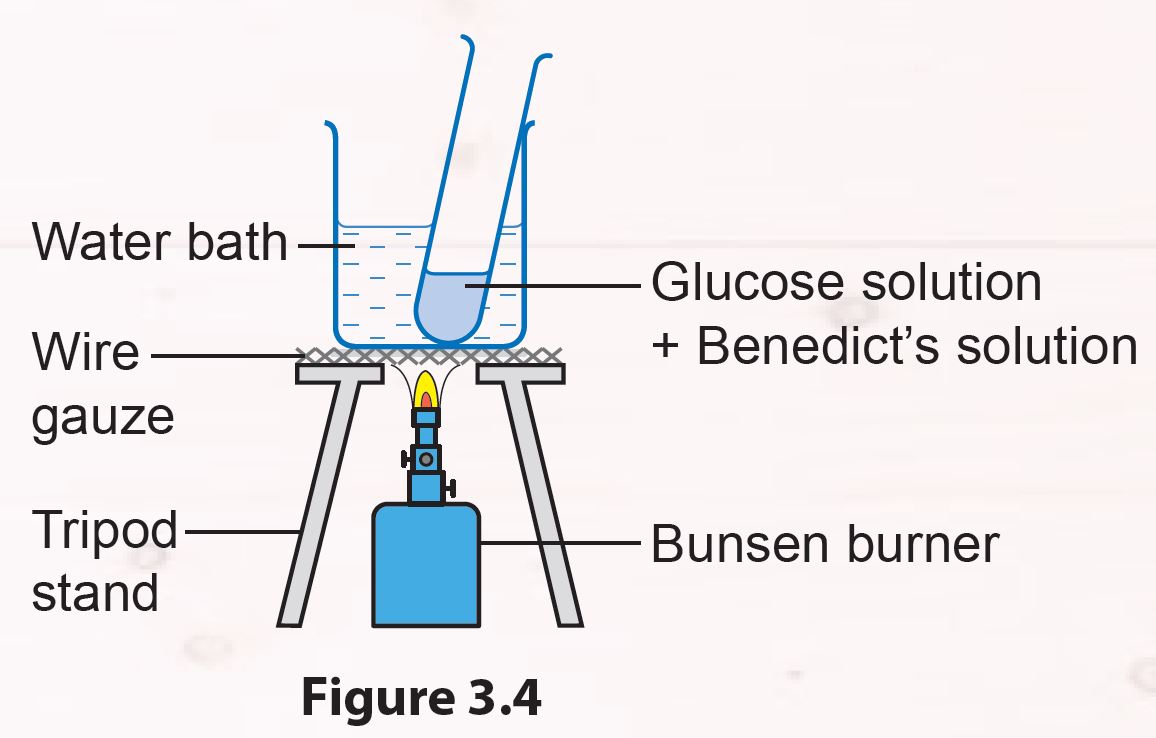
1. Pour 2 ml of glucose solution into a test tube.
2. Add 2 ml of Benedict’s solution into the test tube and shake it to mix the solutions.
3. Heat the test tube in a water bath for 5 minutes (Figure 3.4).
4. Record your observations.
Instruction
1. Pour 2 ml of glucose solution into a test tube.
2. Add 2 ml of Benedict’s solution into the test tube and shake it to mix the solutions.
3. Heat the test tube in a water bath for 5 minutes (Figure 3.4).
4. Record your observations.

(C) Millon’s test for protein:
Instruction
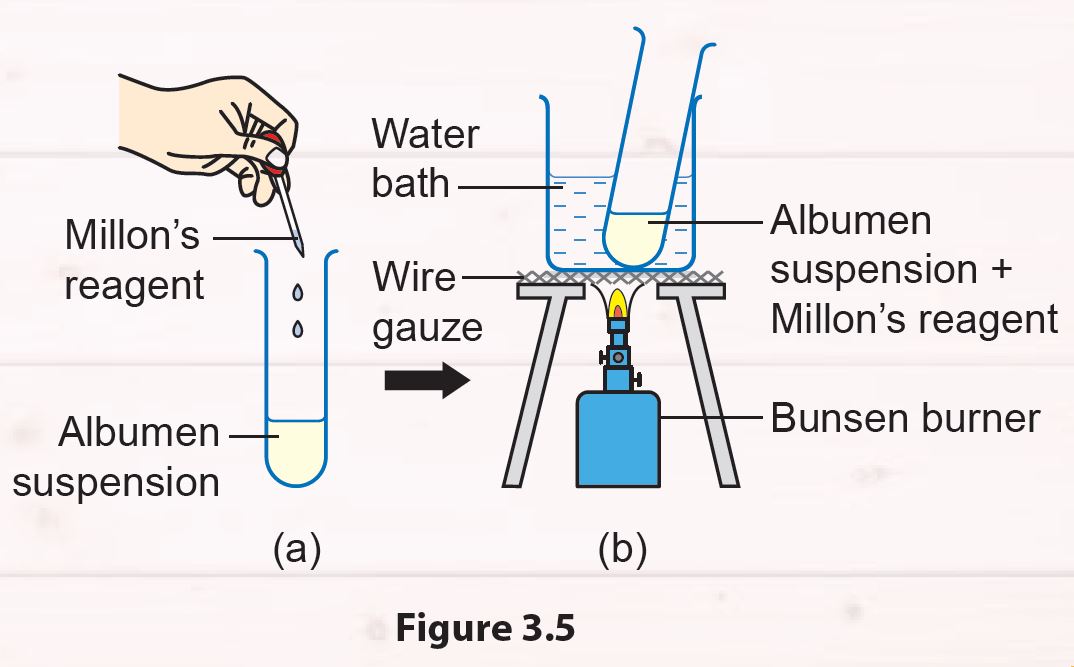
1. Pour 5 ml of albumen suspension into a test tube.
2. Add two to three drops of Millon’s reagent into the test tube (Figure 3.5 (a)). Then, shake the test tube to mix the solutions.
3. Heat the test tube in a water bath for 5 minutes (Figure 3.5 (b)).
4. Record your observations.
Instruction
1. Pour 5 ml of albumen suspension into a test tube.
2. Add two to three drops of Millon’s reagent into the test tube (Figure 3.5 (a)). Then, shake the test tube to mix the solutions.
3. Heat the test tube in a water bath for 5 minutes (Figure 3.5 (b)).
4. Record your observations.

(D) Alcohol-emulsion test for fat:
Instruction
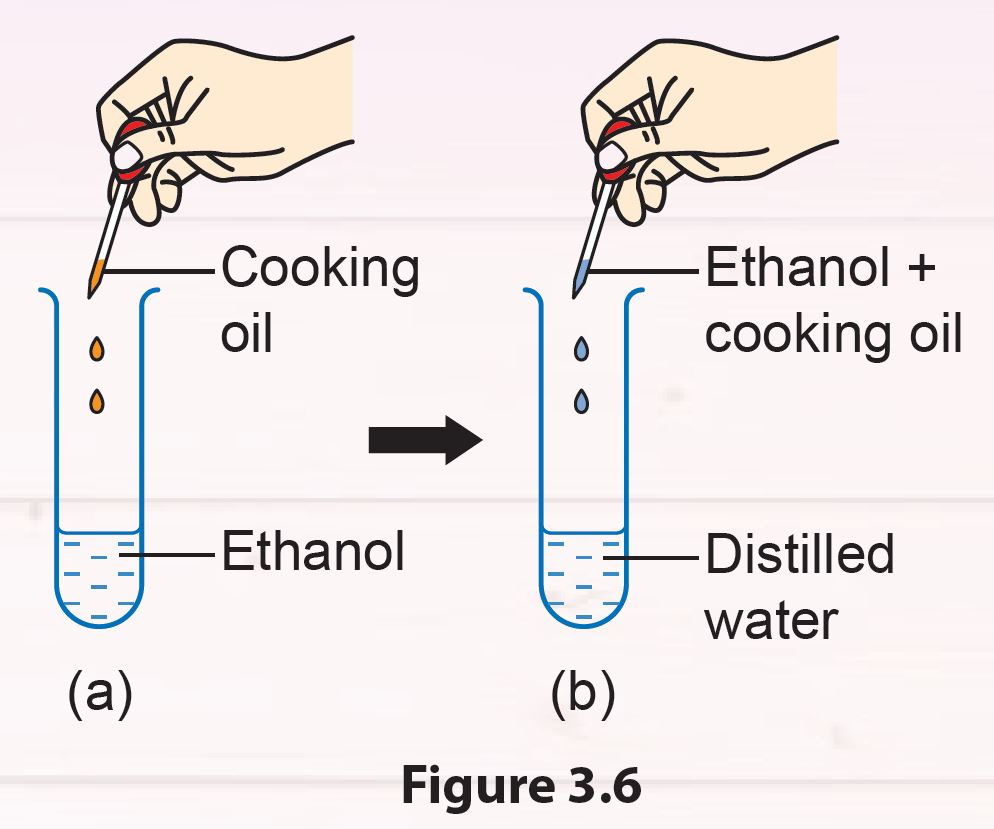
1. Pour 3 ml of ethanol into a test tube.
2. Add 2 to 3 drops of cooking oil into the test tube (Figure 3.6 (a)). Then, close the test tube with a stopper.
3. Shake the test tube slowly and leave it in a test tube rack for 2 to 3 minutes.
4. Place 4 to 5 drops of the mixture from the test tube into another test tube filled with 20 ml of distilled water (Figure 3.6 (b)).
5. Close the test tube with the stopper and shake slowly. Leave the test tube in the test tube rack for 2 to 3 minutes.
6. Record your observations.
Instruction
1. Pour 3 ml of ethanol into a test tube.
2. Add 2 to 3 drops of cooking oil into the test tube (Figure 3.6 (a)). Then, close the test tube with a stopper.
3. Shake the test tube slowly and leave it in a test tube rack for 2 to 3 minutes.
4. Place 4 to 5 drops of the mixture from the test tube into another test tube filled with 20 ml of distilled water (Figure 3.6 (b)).
5. Close the test tube with the stopper and shake slowly. Leave the test tube in the test tube rack for 2 to 3 minutes.
6. Record your observations.

Observation:
Questions:
1. Why is the heating in the Benedict’s is test and Millon’s test carried out in a water bath?
2. You are given a food sample in powder form. How do you determine the food class of the food sample?
3. What are the inferences you can make from each of the activity above?

Questions:
1. Why is the heating in the Benedict’s is test and Millon’s test carried out in a water bath?
2. You are given a food sample in powder form. How do you determine the food class of the food sample?
3. What are the inferences you can make from each of the activity above?
Answer:
1. To make sure the samples are at a constant temperature.
2. First, mix the food sample in powder form with distilled water. Then, carry out the Iodine, Benedict, Millon and alcohol emulsion tests.
3.
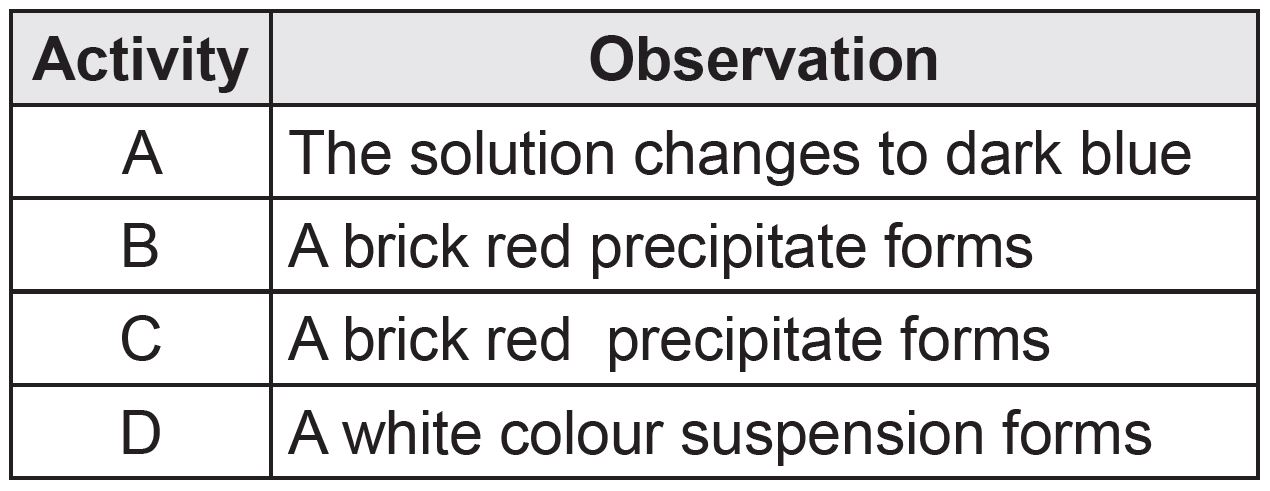
Activity A: The sample will change colour to dark blue because of the presence of starch.
Activity B: A brick red precipitate will form because of the presence of reducing sugar.
Activity C: A brick red precipitate will form because of the presence of protein.
Activity D: A white colour suspension will form because of the presence of fat.

1. To make sure the samples are at a constant temperature.
2. First, mix the food sample in powder form with distilled water. Then, carry out the Iodine, Benedict, Millon and alcohol emulsion tests.
3.
Activity A: The sample will change colour to dark blue because of the presence of starch.
Activity B: A brick red precipitate will form because of the presence of reducing sugar.
Activity C: A brick red precipitate will form because of the presence of protein.
Activity D: A white colour suspension will form because of the presence of fat.