Activity 5.5:
Aim: To prepare dilute solution, concentrated solution and saturated solution.
Materials: Distilled water and copper(II) sulphate crystal
Apparatus: Beaker, measuring cylinder, glass rod and spatula
Instruction
1. Fill three beakers labelled as P, Q and R with 50 ml of distilled water (Figure 5.13).
2. Add two spatulas of copper(II) sulphate crystals into beaker P and stir until all the copper(II) sulphate crystals dissolve.
3. Add four spatulas of copper(II) sulphate crystals into beaker Q and stir until all the copper(II) sulphate crystals dissolve.
4. Add four spatulas of copper(II) sulphate crystals into beaker R and stir until all the copper(II) sulphate crystals dissolve. Add more copper(II) sulphate crystals little by little until excess copper(II) sulphate crystals deposit at the bottom of the beaker.
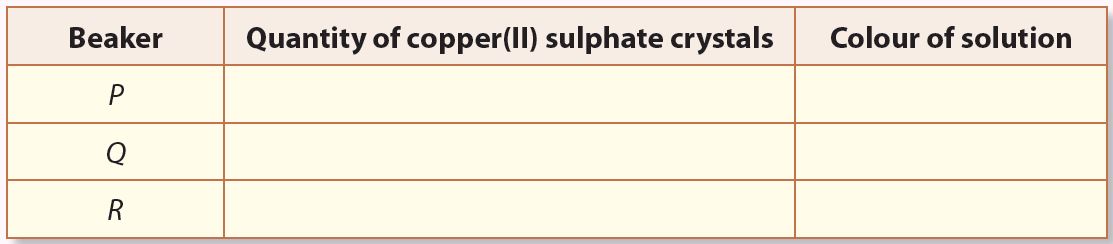
5. Observe all the three beakers and record your observations.
Observation
Questions
1. Identify the types of solutions formed in beakers P, Q and R.
2. Name the solute, solvent and solution used in this activity.
3. Why does a precipitate form in beaker R?
Answer:
1.
P: Dilute solution
Q: Concentrated solution
R: Saturated solution
2.
Solute – Copper(II) sulphate
Solvent – Distilled water
Solution – Copper(II) sulphate solution
3. Copper(II) sulphate has dissolved to the maximum in the distilled water. Thus, the excess copper(II) sulphate remains as a precipitate.
Aim: To prepare dilute solution, concentrated solution and saturated solution.
Materials: Distilled water and copper(II) sulphate crystal
Apparatus: Beaker, measuring cylinder, glass rod and spatula
Instruction
1. Fill three beakers labelled as P, Q and R with 50 ml of distilled water (Figure 5.13).

2. Add two spatulas of copper(II) sulphate crystals into beaker P and stir until all the copper(II) sulphate crystals dissolve.
3. Add four spatulas of copper(II) sulphate crystals into beaker Q and stir until all the copper(II) sulphate crystals dissolve.
4. Add four spatulas of copper(II) sulphate crystals into beaker R and stir until all the copper(II) sulphate crystals dissolve. Add more copper(II) sulphate crystals little by little until excess copper(II) sulphate crystals deposit at the bottom of the beaker.
5. Observe all the three beakers and record your observations.
Observation

Questions
1. Identify the types of solutions formed in beakers P, Q and R.
2. Name the solute, solvent and solution used in this activity.
3. Why does a precipitate form in beaker R?
Answer:
1.
P: Dilute solution
Q: Concentrated solution
R: Saturated solution
2.
Solute – Copper(II) sulphate
Solvent – Distilled water
Solution – Copper(II) sulphate solution
3. Copper(II) sulphate has dissolved to the maximum in the distilled water. Thus, the excess copper(II) sulphate remains as a precipitate.