Activity 6.11:
Aim: To produce a compound
Materials and apparatus: Sulphur powder, iron powder, Bunsen burner, crucible with lid, tripod stand, pipeclay triangle, weighing balance
Procedure
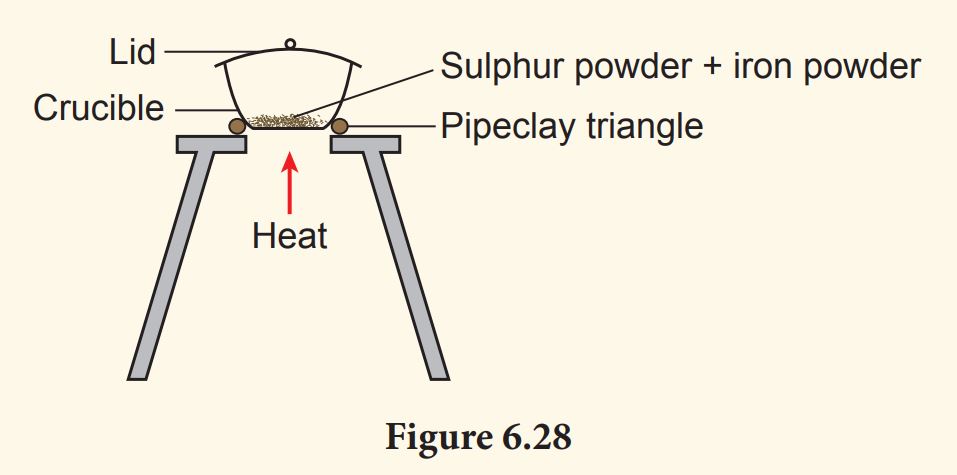
1. Put one spatula of sulphur powder and one spatula of iron powder into the crucible. Stir well. Record the colour of the mixture.
2. Weigh the mixture. Record the initial mass.
3. Heat the mixture until the colour changes (Figure 6.28).
4. Let the product cool. Weigh and record the final mass of the product.
Questions
1. What is the colour of the mixture when it is heated?
2. Write the word equation of the chemical reaction that occurred.
3. What is the product of this reaction?
4. Is there any change in the mass of the compound before and after heating?
Answer:
1. Black
2. Iron + sulphur → iron sulphide
3. Iron sulphide
4. No
Aim: To produce a compound
Materials and apparatus: Sulphur powder, iron powder, Bunsen burner, crucible with lid, tripod stand, pipeclay triangle, weighing balance
Procedure

1. Put one spatula of sulphur powder and one spatula of iron powder into the crucible. Stir well. Record the colour of the mixture.
2. Weigh the mixture. Record the initial mass.
3. Heat the mixture until the colour changes (Figure 6.28).
4. Let the product cool. Weigh and record the final mass of the product.
Questions
1. What is the colour of the mixture when it is heated?
2. Write the word equation of the chemical reaction that occurred.
3. What is the product of this reaction?
4. Is there any change in the mass of the compound before and after heating?
Answer:
1. Black
2. Iron + sulphur → iron sulphide
3. Iron sulphide
4. No