Experiment 5.1:
Problem statement: What is the difference in the rate of diffusion between solids and liquids?
Hypothesis: The rate of diffusion is low in a solid, but high in a liquid.
Aim: To determine the rate of diffusion of copper(II) sulphate in two states of matter.
Variables
(a) Constant variable: Temperature
(b) Manipulated variable: Diffusion medium
(c) Responding variable: Rate of diffusion
Materials and apparatus: Copper(II) sulphate crystals, colourless gel, distilled water, test tube, rubber stopper, measuring cylinder, stopwatch.
Procedure
(A) Diffusion in a solid
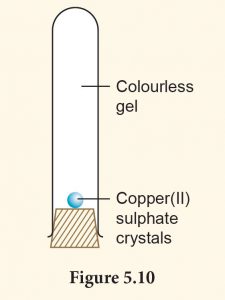
1. Set up the apparatus as shown in Figure 5.10.
2. Observe the changes after two days.
3. Record your observation.
(B) Diffusion in a liquid
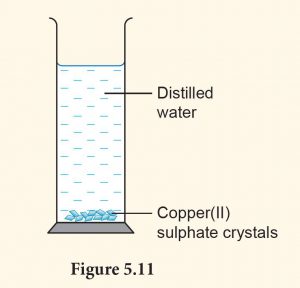
1. Put in one spatula of copper(II) sulphate crystals into a measuring cylinder filled with 50 ml of distilled water (Figure 5.11).
2. Observe the changes after 15 minutes.
3. Record your observation.
Result
Conclusion
Is the hypothesis accepted?
Questions
1. State the observation for Activity A and B.
2. Compare the rate of diffusion of copper(II) sulphate in a solid and a liquid.
Answer:
The rate of diffusion is faster in liquids than in solids.
Problem statement: What is the difference in the rate of diffusion between solids and liquids?
Hypothesis: The rate of diffusion is low in a solid, but high in a liquid.
Aim: To determine the rate of diffusion of copper(II) sulphate in two states of matter.
Variables
(a) Constant variable: Temperature
(b) Manipulated variable: Diffusion medium
(c) Responding variable: Rate of diffusion
Materials and apparatus: Copper(II) sulphate crystals, colourless gel, distilled water, test tube, rubber stopper, measuring cylinder, stopwatch.
Procedure
(A) Diffusion in a solid
1. Set up the apparatus as shown in Figure 5.10.
2. Observe the changes after two days.
3. Record your observation.

(B) Diffusion in a liquid
1. Put in one spatula of copper(II) sulphate crystals into a measuring cylinder filled with 50 ml of distilled water (Figure 5.11).
2. Observe the changes after 15 minutes.
3. Record your observation.

Result

Conclusion
Is the hypothesis accepted?
Questions
1. State the observation for Activity A and B.
2. Compare the rate of diffusion of copper(II) sulphate in a solid and a liquid.
Answer:
The rate of diffusion is faster in liquids than in solids.